Hemoglobin's Oxygen-binding Curve Exhibits a Shape Best Described as
A fetal hemoglobin is composed of 2 alpha and 2 gamma subunits. The pulse oximeter measures the amount of oxygen present in the blood.
Why Is The Oxygen Dissociation Curve S Shaped Quora
Myoglobin contains a heme prosthetic group that can reversibly bind to oxygen.

. The shape of the myoglobin binding curve that shows that its not regulated allosterically. Describe how oxygen is bound to hemoglobin and transported to body tissues. The curve is a valuable aid in understanding how the blood carries and releases oxygen and it is a common theme that is tested on in many medical examinations.
As one oxygen molecule binds hemoglobins affinity for additional oxygen increases and its percent saturation rapidly increases. B Hemoglobin exhibits a hyperbolic O2 saturation curve while myoglobin exhibits a sigmoid shaped curve. All of the above.
D Hemoglobin exhibits a higher degree of O2 saturation at all physiologically relevant partial pressures of O2 than does myoglobin. Ferrous Fe2 Under normal conditions the heme iron in myoglobin and hemoglobin is in the _____ oxidation state. The graph shows the oxygen-binding curves for myoglobin and hemoglobin.
The oxygen-binding curve of hemoglobin does not look like a simple binding curve such as that for myoglobin. Cooperative This condition is a result of a single point mutation in the Beta chain of hemoglobin. The titration curve of myoglobin with oxygen is a hyperbola as shown in Figure of the form.
B Hemoglobin exhibits a hyperbolic O2 saturation curve while myoglobin exhibits a sigmoid-shaped curve. Show that hemoglobin reaches half saturation in the peripheral tissues. It encodes a single polypeptide chain with one oxygen binding site.
For hemoglobin this shape suggests that the binding of oxygen at one site within the hemoglobin tetramer increases the likelihood that oxygen binds at the remaining unoccupied sites. C Hemoglobin exhibits cooperative binding of O2 while myoglobin does not. A concerted model B Michaelis-Menten model.
Where Y is the fraction of oxygenated myoglobin p O 2 is the partial pressure of O 2 expressed in torr mm Hg. MB is the gene encoding myoglobin in humans. Hemoglobin binding of oxygen is best described as a.
They exhibit different oxygen binding curves when plotted on a graph with total partial pressure of the oxygen x-axis plotted against the percent saturation of hemoglobin y-axis. Hemoglobin transports oxygen and myoglobin stores oxygen. The body uses it as an oxygen storage protein in muscle.
After the binding of the first oxygen the hemoglobin changes shape and the binding of additional oxygens is facilitated giving an S-shaped curve. Which of the following statements describes the oxygen binding curve of hemoglobin. Cooperative binding ensures adequate oxygen transport and.
The oxygen-hemoglobin saturation curve illustrates the effect of increasing PO 2 on the percent saturation of hemoglobin with oxygen. Hemoglobin-binding of oxygen is best described as a. E All of the above.
D Hemoglobin exhibits a higher degree of O2 saturation at all physiologically relevant partial pressures of O2 than does myooglobin. Myoglobin is a protein located primarily in the striated muscles of vertebrates. Hemoglobin haemoglobin BrE from the Greek word αἷμα haîma blood Latin globus ball sphere -in ˌ h iː m ə ˈ ɡ l oʊ b ɪ n ˈ h ɛ m oʊ ˌ- abbreviated Hb or Hgb is the iron-containing oxygen-transport metalloprotein in red blood cells erythrocytes of almost all vertebrates the exception being the fish family Channichthyidae as well as the tissues of some.
The oxygen dissociation curve is a graph that plots the proportion of haemoglobin in its oxygen-laden saturated form on the vertical axis against the partial pressure of oxygen on the horizontal axis. When oxygen binds to the first subunit of deoxyhemoglobin it increases the affinity of the remaining subunits for oxygen. Each of the four oxygens bind with equal facility The binding of the first oxygen molecule enhances the binding of the other three oxygen molecules The binding of the first oxygen molecule makes the binding of the other three oxygen molecules more difficult The.
This type of binding is indicated by a sigmoidal-shaped binding curve. _____ This type of binding is indicated by a sigmoidal-shaped binding curve. A fetal hemoglobin is composed of 2 alpha and 2 gamma subunits.
Only 15 percent of oxygen in the blood is dissolved directly into the blood itself. This type of binding is known as allosteric binding where binding at one site affects the affinities of the remaining binding sites. It responds to oxygen availability and releases it when partial pressure is low.
D Hemoglobin exhibits a higher degree of O2 saturation at all physiologically relevant partial pressures of O2 than does myoglobin. C Hemoglobin exhibits cooperative binding of O2 while myoglobin does not. C fetal hemoglobin binds oxygen less than HbA at all pO2.
Hemoglobin Pso hemoglobin B C o torr. What is the shape of the oxygen binding curves for hemoglobin and myoglobin. D fetal hemoglobin does not exist in the T-form.
Although oxygen dissolves in blood only a small amount of oxygen is transported this way. It is able to bind and release oxygen. B Hemoglobin exhibits a hyperbolic O2 saturation curve while myoglobin exhibits a sigmoid-shaped curve.
B Hemoglobin exhibits a hyperbolic O2 saturation curve while myoglobin exhibits a sigmoid-shaped curve. B Hemoglobin exhibits a hyperbolic O2 saturation curve while myoglobin. They exhibit different oxygen binding curves when plotted on a graph with total partial pressure of the oxygen x-axis plotted against the percent saturation of hemoglobin y-axis.
The curve of oxygen binding to hemoglobin is sigmoidal typical of allosteric proteins in which the substrate in this case oxygen is a positive homotropic effector. C Hemoglobin exhibits cooperative binding of O2 while myoglobin does not. E All of the above.
Instead it is a _____ shape which indicates a special binding behavior. While myoglobin exhibits a sigmoid-shaped curve C Hemoglobin exhibits cooperative binding of O2 while myoglobin doesnt. B fetal hemoglobin binds 23-BPG more tightly than normal adult hemoglobin.
Hemoglobin versus in mm Hg graphs as an S-shaped curve as a result of cooperative changes in binding strength with saturation. 760 torr 1 atmosphere and P 0 is the partial pressure of O 2 required to bind 50 of the myoglobin molecules. 10 Answer Bank Hemoglobin curve 0 pressure torr Myoglobin curve Saturation 0 10 20 30 40 50 Use the curves to determine the partial pressure of oxygen at 50 saturation for hemoglobin and myoglobin.
The binding of oxygen to Hb shows a sigmoid curve. Label the graph and answer the questions. Hemoglobin exhibits cooperative binding of O2 while myoglobin does not.
Most oxygen985 percentis bound to a protein called hemoglobin and carried to the tissues. Write that cooperative binding produces this sigmoidal shape.
Hemoglobin Myoglobin 4 Dissociation Curves Biochemistry Flashcards Draw It To Know It
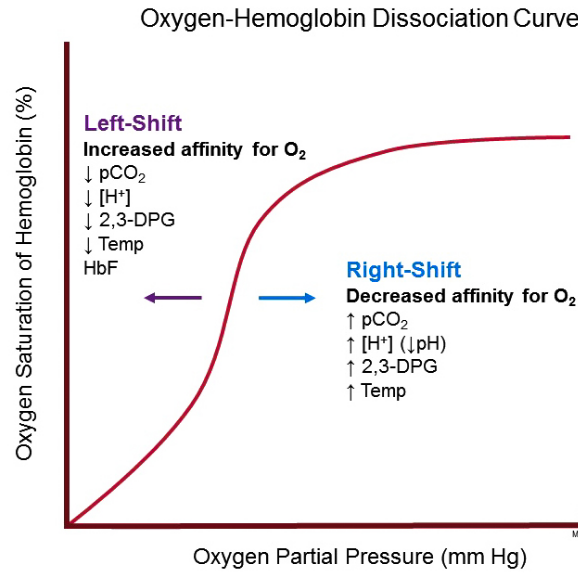
Difference Between Myoglobin And Hemoglobin Oxygen Dissociation Curve With Pictures Viva Differences

Comments
Post a Comment