Oxidation Number of Hydrogen
In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation.
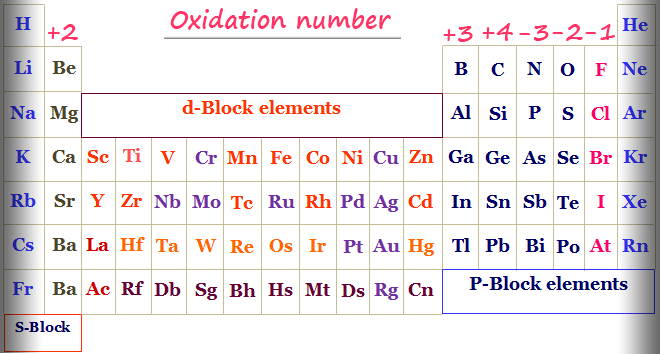
Oxidation Number Periodic Table Elements Definition Rules
For any neutral compound the sum of the oxidation numbers must equal 0.

. The H 2 O 2 is broken down to water by catalase. Oxidation number also called oxidation state is a measure of the degree of oxidation of an atom in a substance see. When iodine chlorine and bromine are combined with oxygen their oxidation.
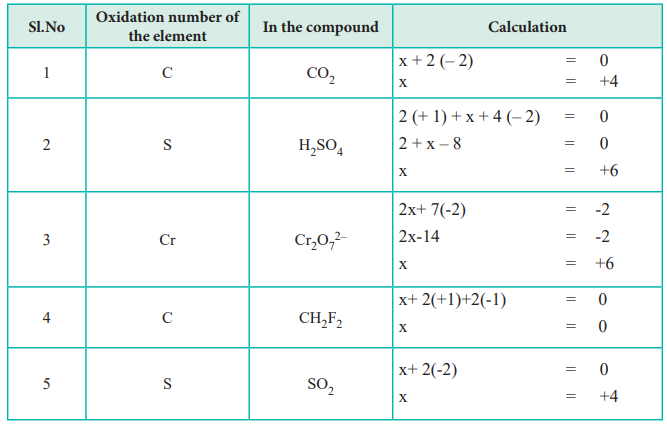
Let the oxidation number of sulphur in H2SO4 be equal to X. The oxidation number of a free element is always 0. The oxidation number of oxygen is 2.
Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it. The number of acetyl-CoA produced depends upon the carbon length of the fatty acid being oxidized. The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.
Advanced Oxidation Process AOP systems. It works by creating powerful fast-acting oxidizers called hydroxyls which are a combination of hydrogen and oxygen atoms found in the earths atmosphere. For example the oxidation number of Na is 1.
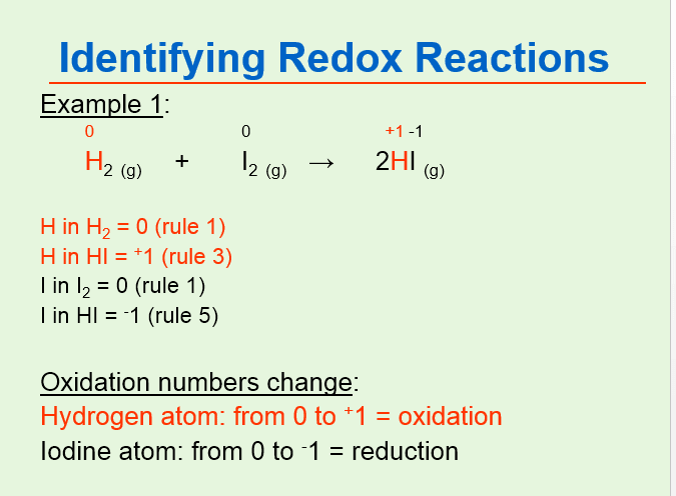
An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron. The oxidation number of hydrogen 1. The term covers a large and diverse body of processes.
The oxidation number of N 3-is -3. Assigning oxidation numbers to organic compounds. As the oxidation state of oxygen 1 in H 2 O 2 sits between molecular oxygen and water there are two possible ways for electrochemical H 2 O 2.
Hydrogen generally has an. The algebraic sum of the oxidation numbers of elements in a compound is zero. How to enter Lot Number COA.
Fluorine and other halogens have an oxidation number 1 when they appear as halide ions in their compounds. The oxidation number of oxygen in a compound is -2 except in peroxides when it is -1. Oxidation and Reduction reactions- The chemical reactions which involve the transfer of electrons from one chemical substance to another.
The oxidation number of oxygen is 2. These electron-transfer reactions are termed as oxidation-reduction reactions or Redox reactions. Now H2 generation during the oxidation of biomass-derived aldehydes is combined.
Hydrogens oxidation number is 1 excluding when it is bonded to metals containing two elements. Cu 2 2 I -1 - Cu 1 I 0 2 b Identify and write out all redox couples in reaction. Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it.
5 100 500 mL in poly bottle. Hydrogen has OS 1 but adopts 1 when bonded as a hydride to metals or metalloids. Enter Lot Number to search for Certificate of Analysis COA.
Herein we report the synthesis of hollow colloidosomes composed of Ru nanocrystals based on a novel gasliquid interface self-assembly strategy. The oxidation number of hydrogen in a compound is 1 except in metal hydrides such as NaH when it is -1. 30 ww in H 2 O contains stabilizer.
Structural characterizations reveal that much defects are. The usual oxidation number of hydrogen is 1. Advanced oxidation or AOP is a newer method in pool oxidation and has generated a lot of excitement recently within the pool industry.
The oxidation number of sulphur in H 2 SO 4 is 6. Hydrogen production from water electrolysis requires high working voltages and produces H2 only at the cathode. The atoms in He and N 2 for example have oxidation numbers of 0.
The oxidation number of a monatomic ion equals the charge of the ion. Redox reactions are common and vital to some of the basic functions of life including photosynthesis respiration combustion and corrosion or rusting. An important difference is acyl-CoA oxidase the first enzyme in peroxisome β-oxidation which transfers the hydrogen to oxygen producing H 2 O 2 instead of producing FADH 2.
Many oxidation-reduction reactions are as common and familiar as fire the rusting and dissolution of metals the browning of fruit and respiration and. Rules for assigning oxidation numbers. Oxidation Number of Chromium in Cr 2 O 2 7 ion.
To learn more about the. For example CaH 2 its oxidation number equals to 1. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change.
Developing efficient hydrogen oxidation reaction HOR electrocatalysts in alkaline media is of great significance for anion exchange membrane fuel cells. The oxidation and reduction reaction also involve the addition of oxygen or hydrogen to different substances. The oxidation number of an atom in elemental form is 0.
Hydrogen has an oxidation number of 1 when combined with non-metals but it has an oxidation number of -1 when combined with metals. All Photos 1 Empirical Formula Hill Notation. Oxidation-reduction reaction also called redox reaction any chemical reaction in which the oxidation number of a participating chemical species changes.
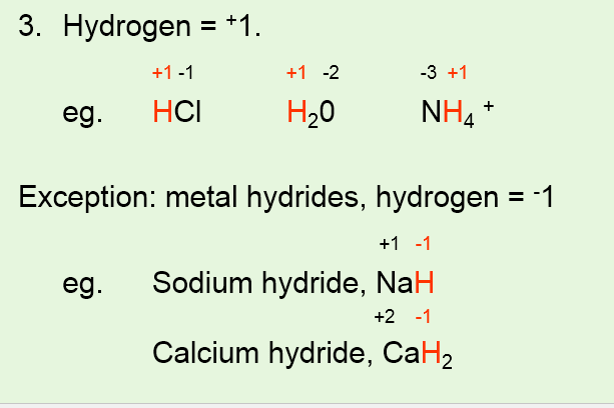
Oxidation Numbers Vce Chemistry

Oxidation Number State Definition Rules How To Find And Examples
Comments
Post a Comment